Abstract
Extensive gene expression profiling and RNA interference studies revealed the frequently chemo-resistant activated B-cell-like (ABC) subtype of diffuse large B-cell lymphoma (DLBCL) relies on constitutive B-cell receptor (BCR) signaling. As such, the clinical importance of BCR signaling inhibition is well appreciated and thus far led to the development of kinase and protease inhibitors. However, this therapeutic approach fails to achieve complete, sustained responses in DLBCL patients because of inherent resistance due to additional genetic lesions in other components of the BCR pathway or acquired kinase mutations. The emerging field of DNA secondary structures support that guanine (G)-rich stretches of DNA capable of adopting G-quadruplex (G4) motifs act as transcription regulatory units, or switches, that can turn gene expression on or off. Targeting G4s is likely to overcome activating kinase mutations by limiting the amount of gene available for translation into protein. Here, we explore a drug discovery effort based on targeting G4 within BCR genes critical for ABC DLBCL cell survival, CD79A, CD79B, CARD11, and MYD88.
We first interrogated the BCR-related genes within the hg19 human reference genome for G-rich DNA using a G4 algorithm and discovered each of the four genes contain G4 forming sequences near promoter regions. These G4 elements formed stable G4 structures as determined by circular dichroism (CD) spectroscopy, the standard for visualizing macromolecule secondary structure formation. Melting curves are also generated from CD spectroscopy to determine the thermal stability of a given structure. The CD79A, CD79B, CARD11, and MYD88 G-rich sequences displayed classic, stable G4 structure spectra consisting of negative minima absorption peaks at 240-265 nm and a positive maximum at 260-295 nm with melting temperatures ranging from 62 to 95 °C.
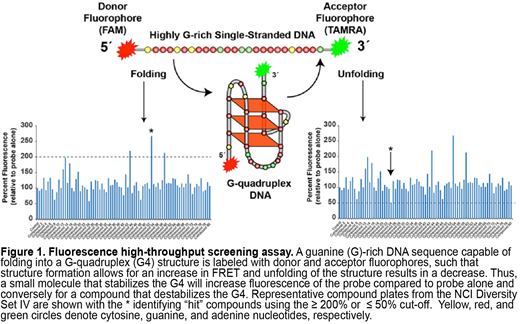
We then developed a high-throughput screening assay based on fluorescence resonance energy transfer (FRET) to identify G4 interactive compounds from the NCI Diversity Set IV library (1584 compounds) that uniquely interact with each of the BCR G4 sequences. This screen used the BCR G4 sequences as molecular bait where the 5´-end and 3´-end of the oligomers were labeled with a FAM- and a TAMRA-fluorophore, respectively, such that G4 formation leads to an increase in fluorescence emission (Figure 1). The initial FRET screen tested compounds at a 1:5 molecular ratio of probe to compound and measured the change in fluorescence relative to probe alone. Overall, the screen resulted in a ~1% "hit" rate for each BCR target, except for CD79B, which yielded a lower percent of interactive compounds (0.3%). Seven compounds, which included ellipticine, quinoline, and daunomycin derivatives, were identified to selectively target the CARD11 (n=3), MYD88 (n=3), or CD79A (n=1) G4s relative to other G4, single-stranded, and double-stranded DNA. Of note, all five compounds found to interact with the CD79B G4 also altered FRET of the other BCR G4 sequences. Subsequent FRET validation and CD analyses where each of the BCR sequences was incubated with increasing concentrations of candidate compounds demonstrated dose-dependent effects on G4 structure formation, particularly stabilization of the CARD11 G4 with compound NCI 9037 that resulted in a 300% FRET increase and an 8 °C shift in melting temperature at a 1:10 ratio.
This study identifies DNA G4 as a new class of molecular targets for inhibiting an important oncogenic pathway. Discovery of selective compounds in addition to those with "pan" interaction, suggests the CARD11, MYD88, and CD79A G4 have unique folding patterns whereas the CD79B G4 may exhibit more common structural features. These compounds will be used as molecular tools to provide further insight into the structures and mechanisms in which G4 regulate gene transcription. In establishing a high-throughput screen, we discovered compounds for which preclinical development is ongoing and includes evaluation of the effects on BCR target gene and protein expression, inhibition of downstream BCR signaling, and consequent ABC DLBCL tumor growth and survival. This treatment strategy has high potential for leading to a breakthrough in effectively targeting the constitutively active molecules and greatly impacting the clinical management of patients with BCR-dependent DLBCL.
No relevant conflicts of interest to declare.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal